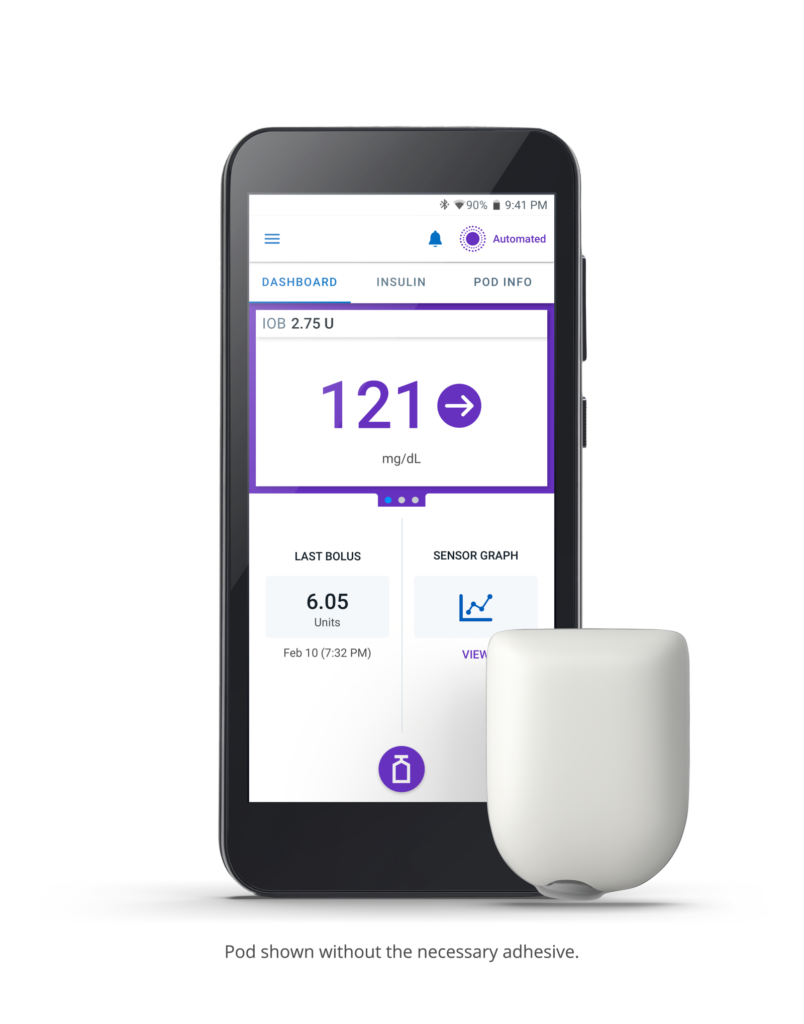
Diabetes care has taken a significant step forward following FDA clearance of Acton-based Insulet’s Omnipod® 5 Automated Insulin Delivery (AID) System. It is the first and only AID system approved for the treatment of both type 1 (formerly called juvenile) diabetes and type 2 (formerly called adult-onset) diabetes.
Over 30 million Americans live with type 2 diabetes in the United States [National Diabetes Statistics Report], with approximately 6 million people requiring insulin and 2.5 million people relying on multiple daily injections. Over the past 30 years, there has been no significant improvement in the percentage of the adult type 2 population achieving recommended blood sugar targets.
This type 2 diabetes management system replaces the daily routine of fingersticks and injection needles with a discreet wearable patch that provides insulin for up to three days. It protects highs and lows both day and night and eliminates the need for multiple daily injections.

Based on data presented recently at the American Diabetes Association (ADA) meeting, the Omnipod 5 patch significantly improved outcomes while making life easier for people with type 2 diabetes. It also reduced the total amount of insulin patients used each day. Omnipod 5 is currently FDA-cleared in the U.S. for people with type 1 diabetes ages two and older as well as type 2 diabetes ages 18 and older.
Insulet’s international headquarters opened its doors at 100 Nagog Park in Acton in 2019. According to a 2019 communique from the Town of Acton, “In 2017 Acton Town Meeting approved a Tax Increment Financing (TIF) plan for Insulet to provide a 100% tax break for the first five years on the significant capital investment at the 100 Nagog Park property, providing a total tax break of 62.5% over a 20 year term.” The company broke ground in Acton in 2017.

Janet Furey is a writer and copy editor for the Acton Exchange.













